Led by the PREVENT-nCoV consortium the trial involved 45 healthy adults with no previous SARS-CoV-2 infection. Biotechnology company Bavarian Nordic AS may sell its Covid-19 vaccine candidate if it cant raise the money needed to carry out trials on humans.

Annual Report 2020 Bavarian Nordic
The company is headquartered in Kvistgaard Denmark a town in Helsingør Municipality where it also operates a commercial-scale manufacturing facility.

Bavarian nordic vaccine. BAVA announced today the initiation of a Phase 2 clinical trial of its. COPENHAGEN Denmark August 23 2021 Bavarian Nordic AS OMX. Bavarian Nordic is a fully integrated vaccines company focused on the development manufacture and commercialization of life-saving vaccines.
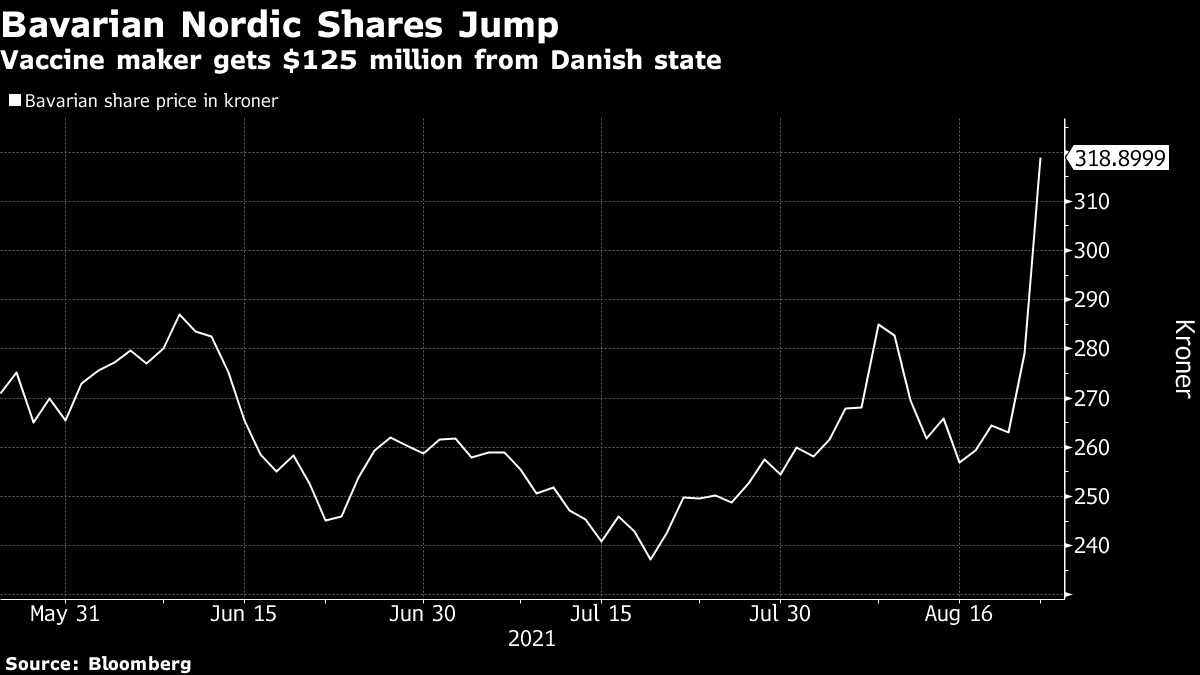
BAVA announced today the initiation of a Phase 2 clinical trial of its COVID-19 vaccine. Bloomberg -- Denmarks government will spend as much as 800 million kroner 125 million to help Bavarian Nordic AS finance its Covid-19 vaccine candidate. COPENHAGEN Denmark August 23 2021 Bavarian Nordic AS OMX.
Paul Chaplin President and CEO of Bavarian Nordic commented. We are a global leader in smallpox vaccines and have been a long-term supplier to the US. Strategic National Stockpile of a non-replicating smallpox vaccine which has been approved by the FDA under the trade name JYNNEOS also for the.
Phase 2 trial will investigate vaccines ability to. It has shown really good results so far Health Minister Magnus Heunicke said in a Copenhagen press briefing on Monday. Bavarian Nordic AS is a fully integrated biotechnology company focused on the development manufacturing and commercialization of cancer immunotherapies and vaccines for infectious diseases.
Bavarian Nordics shares jumped as much as 18 on Monday after the Danish firm reported encouraging data from its COVID-19 vaccine candidate which is. Bavarian Nordic has announced positive preliminary data from the first-in-human Phase III dose-escalation clinical trial of its Covid-19 vaccine ABNCoV2. Initially developed by AdaptVac using its capsid virus-like particle cVLP technology ABNCoV2 was licensed by Bavarian Nordic for worldwide commercialisation and clinical development.
Bavarian Nordic Rises 3 After Covid Vaccine Progresses to Phase 2 PLX AI - Bavarian Nordic shares rose 3 at the open after the company started a phase 2 trial for its Covid-19 booster vaccine. About ABNCoV2 and the Phase 2 trial ABNCoV2 is a next-generation COVID-19 vaccine candidate initially developed by AdaptVac using their proprietary capsid virus like particle cVLP technology. Bloomberg -- Denmarks government will spend as much as 800 million kroner 125 million to help Bavarian Nordic AS finance development of the Danish drugmakers experimental Covid-19 vaccine candidate.
Bavarian Nordic has licensed the global commercialization. Subjects were enrolled at Radboud University Medical Centre in the Netherlands. Bavarian Nordic is a fully integrated vaccines company focused on the development manufacture and commercialization of life-saving vaccines.
Bavarian Nordic joining the project significantly strengthens the Covid-19 cVLP vaccine development effort and ensures the vaccine can reach the public as fast as possible A member of the PREVENT-nCoV consortium AdaptVac secured an EU Horizon grant earlier this year to advance its vaccine candidate into the clinic. Bavarian Nordic will further advance the vaccine candidate and has planned a Phase 2 trial in up to 210 subjects with an aim of in addition to confirming Phase. In parallel with the Phase 2 trial Bavarian Nordic is preparing for a Phase 3 trial of ABNCoV2 in 2022 pending external funding.
It has shown really good results so far Health Minister Magnus Heunicke said in a Copenhagen press briefing on Monday. PLX AI Bavarian Nordic Initiates Phase 2 Clinical Trial of COVID-19 Booster Vaccine. We are pleased to advance our COVID-19 vaccine candidate into Phase 2 that.
Sponsored by Bavarian Nordic the new Phase II trial is designed to enrol up to 210 healthy adults. Denmarks government will spend as much as 800 million kroner 125 million to help Bavarian Nordic AS finance its Covid-19 vaccine candidate. In parallel with the Phase 2 trial Bavarian Nordic is preparing for a Phase 3 trial of ABNCoV2 in 2022 pending external funding.
Janssen Enters Collaboration With Bavarian Nordic To Develop Vaccines Against Hepatitis B Virus And Hiv 1

University Of Copenhagen Spinout Enters License Agreement With Bavarian Nordic Nordic Life Science The Leading Nordic Life Science News Service
Cgmp Facility Revamp Secures Fast Vaccine Supply Nne
Http Www Bavarian Nordic Com Media 286947 Rabies Fact Sheet Pdf

Bavarian Nordic Covid 19 Vaccine Yields Positive Phase I Ii Data
Http Www Bavarian Nordic Com Media 286947 Rabies Fact Sheet Pdf

Bavarian Nordic Buys Travel Vaccine Brands From Gsk
J J Vaccine Recipients Seek Mrna Booster Without Cdc S Blessing Bloomberg
Bavarian Nordic S Smallpox Vaccine Fda Approved To Fight Bioterrorism

Bavarian Nordic Covid 19 Vaccine Yields Positive Phase I Ii Data

Bavarian Nordic Reports Encouraging Data For Covid 19 Vaccine Candidate

Bavarian Saetter Gang I Fase 2 Studie Med Covid 19 Vaccine

Bavarian Nordic Wraps Up Enrollment Of Last Imvamune Study To Support Fda Nod Fiercepharma